A Researcher Proposes a Model of an Enzyme
Click on the mouse at left to clear the text and images. Based on the model which of the following statements best explains an enzymes specificity for a particular substrate molecule.
The induced fit model is a model for the interaction of enzymes and substrates.
. Penicillin G acylases are intracellular enzymes found in E. These models incorporate the combined influences of. The model is based on the idea that the reactant passes through a transition state within the enzyme-substrate complex before the reactant is converted to the product.
A kinetic model with effect of water content for enzyme-catalyzed citronellyl laurate was developed. According to the induced fit model proposed by Daniel Koshland in 1958 the active site changes until the substrate is entirely bonded to the enzymes active site at which time the final shape and charge are established. In model B AChE is added to the cytoplasm of the postsynaptic cell.
Model of the molecular complex that results when the blood plasma protein fetuin-B purple and pink binds with the enzyme meprin β other colors Credit. Meantime some reports show that PEF treatment has no effect on inactivation of certain. Binding of the substrate to the enzyme changes the configuration of both so that they fit together.
8 ENZYMES MECHANISM OF ACTION OF ENZYMES 1-Lock. Here we presented a three-state model for describing thermal deactivation of Photinus pyralis that shares some common elements with the previously proposed three-state Equilibrium model. A hydrophilic molecule interacts with nonpolar side chains in the enzymes active site.
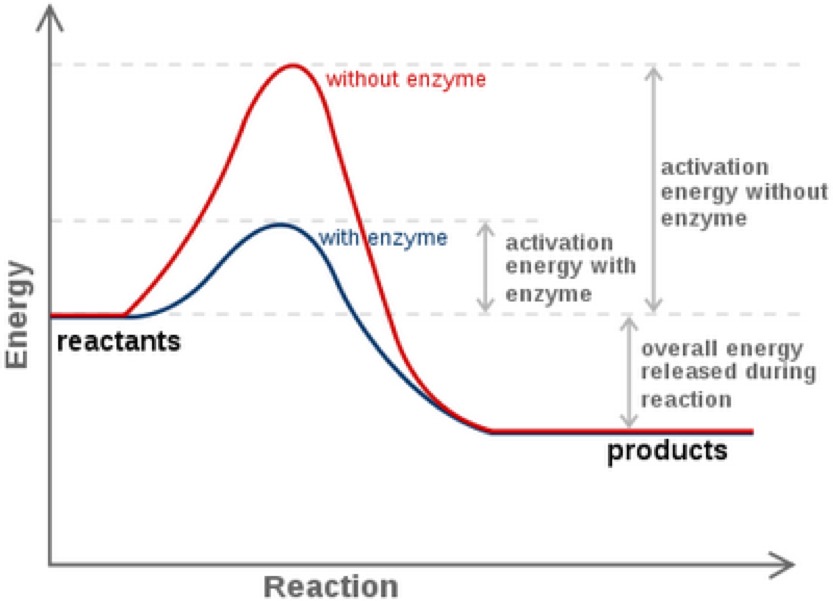
A researcher proposes a model of an enzyme-catalyzed reaction in which a reactant is converted to a product. The effect of pH on three different enzymes. For many years scientists thought that enzyme-substrate binding took place in a simple lock-and-key fashion.
A researcher proposes a model of an enzyme-catalyzed reaction in which a reactant is converted to a product. The model is based on the idea that the reactant passes through a transition state within the enzyme-substrate complex before the reactant is converted to the productWhich of the following statements best helps explain how. Researchers at Johannes.
The shape of the enzyme molecule and the substrate molecule should fit each other like a lock and Key 2. The researcher proposes two models A and B for using acetyl-cholinesterase an enzyme that degrades acetylcholine to prevent the effect of the neurotoxin. To test the claim the researcher carries out an experiment that includes three different enzymes.
In model A AChE is added to the synapse. This model asserted that the enzyme and substrate fit together perfectly in one instantaneous step. A model for enzymesubstrate interaction states that only the right substrate may cause the active site to align properly allowing the enzyme to execute its catalytic activity.
In the past 20 years several studies have confirmed that PEF at high intensity or in combination with mild heat causes substantial inactivation of several food quality-related enzymes such as alkaline phosphatase peroxidase proteases lipase pectin methylesterase polyphenol oxidase etc. Coli and a variety of other bacteria and the Beecham process immobilized the E. Medical lab Tech Biochemistry Lecture.
However current research supports a more refined view called induced fit. The overall goal of this research proposal is to develop an enzyme that can degrade microplastics for application in wastewater treatment. Key model of enzyme action implies that the active site of the enzyme is complementary in shape to that of its substrate ie.
As a model system we will focus on engineering of IsPETase which can degrade PET microplastics so that it can self-assemble onto silica sand particles while preserving its active site. The results of the experiment are represented in Figure 1. A researcher proposes a model of an enzyme-catalyzed reaction in which a reactant is converted to a product.
A researcher proposes a model to explain how enzyme-substrate interactions determine enzyme specificity. The development of immobilized penicillin G acylase dates back to research conducted in 1969 by University College London and Beecham Pharmaceuticals in the UK. The model is based on the idea that the reactant passes through a transition state within the enzyme-substrate complex before the reactant is converted to the product.
Coli enzyme on a DEAE ion-exchange support. Click on the numbers below to see how the induced fit model of enzyme action works. The model is based on the idea that the reactant passes through a transition state within the enzyme-substrate complex before the reactant is converted to the productWhich of the following statements best helps explain how the enzyme speeds.
As the enzyme and substrate come together their interaction causes a mild shift in the enzymes structure that confirms an ideal binding arrangement between the enzyme and the substrate. Play this game to review Biology. Finally it is proposed that a re-examination of many current aspects in enzyme structure and function specifically protein folding x-ray and NMR structure analyses.
A researcher claims that different enzymes exhibit maximal function over different pH ranges. Before binding the substrate and enzyme do not exactly fit each other. Pepsin salivary amylase and arginase.
The model is based on the idea that substrate molecules form favorable interactions with the amino acid side chains in an enzymes active site. However current research supports a more refined view called induced fit. The model is based on the idea that substrate molecules form favorable interactions with the amino acid side chains in an enzymes active site.
A researcher proposes a model of an enzyme-catalyzed reaction in which a reactant is converted to a product. The results of our study as well as previous literature reports indicate that the conventional two-state model of enzyme deactivation needs to be revised. However there are also important.
The induced fit model in contrast to the lock-and-key paradigm demonstrates that enzymes are very flexible structures. It states that only the appropriate substrate may cause the active site to align properly. An alternate model for general enzyme catalysis the Shifting Specificity model is reintroduced and an assessment of why it may be superior to the Haldane model is presented.
This model asserted that the enzyme and substrate fit together perfectly in one instantaneous step. Based on the model which of the following statements best explains an enzymes specificity for a particular. As the enzyme and substrate come together their interaction causes a mild shift in the enzymes.
Predict the effectiveness of each proposed model.

Happy Birthday To Maud Menten 1879 1960 Biochemist And Medical Researcher Co Author Of The Famous Michaelis Menten Equation In Linocut Enzyme Kinetics Maud


0 Response to "A Researcher Proposes a Model of an Enzyme"
Post a Comment